Translate this page into:
Osteoid containing breast masses: A rad-path correlation

*Corresponding author: Purvi Haria, Department of Radiology, Tata Memorial Hospital, Mumbai, Maharashtra, India. drpurvianand@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Palsetia DR, Shet T, Haria P, Thakur M, Kulkarni SS, Thakkar PB, et al. Osteoid containing breast masses: A rad-path correlation. Indian J Breast Imaging 2023;1:6-14.
Abstract
Objectives:
Breast cancer is very common, of which the most common subtype is invasive ductal cancer (IDC). However, there are some rare types that also occur and manifest radiologically in a different manner.
Our article aims to emphasize a rare type of breast neoplasm, which shows osteoid matrix on pathology, and to correlate their imaging findings, thus enabling differentiation from usual carcinoma and aiding in further pathology typing.
Material and Methods:
A retrospective audit of breast masses with osteoid matrix was done from 2008 to 2021. The cases were retrieved from pathology archives. Twenty-five such cases were found with histopathology reports mentioning the osteoid matrix. Eight patients from these 25 had pre-operative mammograms as well as histopathology available for review. We evaluated these with mammograms with multiple parameters.
The project was approved by the Institutional Ethics Committee. Waiver of consent was obtained since there was no direct contact between the researcher and the participants.
Results:
Most cases of eccentric coarse calcification seen on mammography showed type IV matrix, in turn meaning that these cases had mature bone formation with tumor cell rimming type on histopathology.
Three tumors produced an osteoid matrix in our study, namely, Metaplastic carcinoma, primary osteosarcoma, and malignant phyllodes tumor.
Coarse calcification mostly indicates metaplastic carcinoma with osteosarcoma element. The preoperative diagnosis can guide pathology sampling.
Conclusion:
When eccentric, large, and coarse calcifications are visible within a mass on a mammogram, it raises suspicion of mature bone formation with tumor cell rimming within the tumor matrix.
Keywords
Breast cancer
Osteoid
Radiology
Pathology
Introduction
Less than 5% of breast cancers are metaplastic cancers and sarcomas.[1] Some of these might have a presence of an osteoid matrix within them. According to the literature, osteoid matrix is present in 0.2% of breast cancers.[1] In this retrospective audit, we analyzed cases of osteoid-containing neoplasms on mammography and correlated them with histopathology features. The aim of this study was to identify if there is any correlation between mammography findings of calcifications and histopathology of lesions.
Material and methods
A retrospective audit of breast masses with osteoid matrix was performed from 2008 to 2021. The cases were retrieved from pathology archives. Twenty-five such cases were found with histopathology reports mentioning the osteoid matrix. Of these, eight patients had preoperative mammograms as well as histopathology images available for review, and we evaluated them with multiple parameters as described below.
On evaluation of these mammograms, calcifications were divided depending on the morphology seen on the mammogram. As there is no such grading available in the literature, we divided these calcifications depending on our observational finding of mammographic appearance. The working classification we devised is as follows:
No calcifications on the mammogram.
Thin mesh-like calcifications [Figure 1a]: Calcifications are present in a thin mesh-like pattern involving almost the entire tumor.
Scanty coarse calcifications [Figure 1b]: Discrete coarse calcifications involving part/entire of the tumor.
Eccentric large coarse calcifications [Figure 1c]: Dense calcifications/ossification, limited to a part of a tumor, with relative sparing of the rest of the tumor.
- Representative image of thin mesh-like calcifications (white arrows).

- Representative images of scanty coarse calcifications (white arrows).

- Representative images of eccentric large coarse calcifications (white arrows).
Mammograms were also evaluated for the size of the lesions, satellite lesions, and the presence of lymph nodes.
Subsequently, these cases were also evaluated on histopathology in a blinded manner. The amount of osteoid matrix within the tumor was graded visually and classified into four types:
Type I – Hyaline thin filigree strands, non-calcified [Figure 2a].
Type II – Thin strands with thicker bands with osteoblast rimming calcification [Figure 2b].

- Representative histopathological image of Type I osteoid matrix.

- Representative histopathological image of Type II osteoid matrix.
Type III – Chondromyxoid matrix deposition [Figure 2c].

- Representative histopathological image of Type III osteoid matrix.

- Representative histopathological image of Type IV osteoid matrix.
Type IV – Bone formation with tumor cell rimming [Figure 2d].
The final histological diagnosis was based on the panel of immunohistochemistry, which included AE1/AE3, CD34, SMA, and desmin as required.
Once the independent evaluation of mammogram and histopathology was performed in a blinded manner, a combined evaluation of these cases was done.
Results
These patients had varied ages of presentation, the youngest being 34 years and the oldest being 73 years, with 52 years as median.
Two patients had been treated for breast carcinoma in the past and osteoid-forming lesion was seen in the recurrence. One of the patients had recurrence in the same breast and the other in the opposite breast.
On mammography, with respect to breast density, scattered fibroglandular parenchyma was seen in four patients, whereas heterogeneously dense breast parenchyma was seen in three cases and extremely dense parenchyma was seen in one case.
The average size of the tumor measured in the cranio-caudal view (CC) view on mammogram was in the range of 3.5 to 4 cm, with the size of the largest tumor being 7.3 cm.
None of the cases showed satellite nodules or suspicious axillary lymph nodes on the mammogram.
A summary of the results is provided in Table 1.
| Sr. No. | Age (years) | Prior history of breast cancer | Breast density on mammogram | Size of mass on mammogram (measured on CC view) | Calcifications on mammogram | Type of calcifications on histopathology | Histopathological diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | 73 | Nil | Scattered fibroglandular parenchyma | 3.5 × 3 cm | No calcifications | I | Metaplastic carcinoma with chondroid matrix and hyaline material |
| 2 | 52 | Nil | Scattered fibroglandular parenchyma | 5 × 4.5 cm | Eccentric large coarse | IV | Osteosarcoma areas with areas of microglandular adenosis and hence labeled as metaplastic carcinoma |
| 3 | 50 | BCT for ipsilateral breast mass | Scattered fibroglandular parenchyma | 3.5 × 3 cm | Eccentric large coarse | IV | Osteosarcoma areas with foci of DCIS and hence labeled as metaplastic carcinoma |
| 4 | 64 | BCT for contralateral breast mass | Scattered fibroglandular parenchyma | 3.5 × 3 cm | Eccentric large coarse | IV |
Primary osteosarcoma (no epithelial element found) |
| 5 | 60 | Nil | Heterogeneously dense fibroglandular parenchyma | 5.2 × 3.6 cm | Eccentric large coarse | III | Malignant phyllodes with foci of osteosarcoma |
| 6 | 34 | Nil | Extremely dense fibroglandular parenchyma | 7.3 × 5.3 cm | Scanty coarse | II | Malignant phyllodes tumor with osteochondroid matrix |
| 7 | 41 | Nil | Heterogeneously dense fibroglandular parenchyma | 3.7 × 3.3 cm | Thin mesh like | II | Malignant phyllodes tumor with osteochondroid matrix |
| 8 | 52 | Nil | Heterogeneously dense fibroglandular parenchyma | 4 × 4 cm | Eccentric large coarse | IV | Primary osteosarcoma |
BCT: Breast conservative therapy, DCIS: Ductal carcinoma in-situ.
Out of eight cases, five cases had eccentric large coarse calcifications on mammograms [Figures 3 and 4]. One case had scanty coarse calcifications [Figure 5]; one had thin mesh-like calcifications [Figure 6].
![Grade 3 osteoid matrix: (a, b) Bilateral cranio-caudal view (CC) and Medio-lateral oblique (MLO) mammograms of a 52-year-old woman showing an oval to round high-density mass with circumscribed margins in the upper outer quadrant of the right breast with adjacent architectural distortion. [magnified view (c)] It shows eccentric coarse calcifications within. (d, e) The histopathology slides of the mastectomy specimen of the patient show grade 3 chondromyxoid matrix with osteoid. (f, g) Bilateral CC and MLO mammograms of a 60-year-old woman shows an irregular high-density mass with obscured margins in the outer central region of the left breast with eccentric large coarse calcifications seen within. A cutaneous lesion is seen in the upper aspect of the right breast. (h, i and j) Histopathological images of the left breast mass post mastectomy shows grade 3 osteoid matrix.](/content/156/2023/1/1/img/IJBI-1-1-6-g0008.png)
- Grade 3 osteoid matrix: (a, b) Bilateral cranio-caudal view (CC) and Medio-lateral oblique (MLO) mammograms of a 52-year-old woman showing an oval to round high-density mass with circumscribed margins in the upper outer quadrant of the right breast with adjacent architectural distortion. [magnified view (c)] It shows eccentric coarse calcifications within. (d, e) The histopathology slides of the mastectomy specimen of the patient show grade 3 chondromyxoid matrix with osteoid. (f, g) Bilateral CC and MLO mammograms of a 60-year-old woman shows an irregular high-density mass with obscured margins in the outer central region of the left breast with eccentric large coarse calcifications seen within. A cutaneous lesion is seen in the upper aspect of the right breast. (h, i and j) Histopathological images of the left breast mass post mastectomy shows grade 3 osteoid matrix.
![Grade 4 osteoid matrix: [a, b and magnified view (c)] Bilateral CC and MLO mammograms of a 66-year-old woman who had undergone right breast conservation therapy in 2003 and developed a left breast malignancy 15 years later. The mammogram shows postoperative changes in the right breast in the form of minimal architectural distortion and skin retraction in the upper outer quadrant of the right breast, which is also smaller than the left. There is an irregular high-density mass with indistinct margins in the upper central region of the left breast with large coarse calcifications seen within. This patient underwent a lumpectomy. [d, e and magnified view (f)] Bilateral CC and MLO view mammograms (d, e) of a 50-year-old woman, who had undergone right breast conservation therapy in 2014 and developed recurrence in the same breast 7 years later. The mammogram shows the postoperative changes in the right breast with scar site dystrophic calcifications in the lower central region in the retromammary zone (arrows). There is an irregular high-density mass (shouldered arrows) with obscured margins in the upper outer quadrant of the right breast with coarse eccentric calcifications. This patient underwent completion mastectomy. (g, h and i) Histopathological images of the breast masses post-surgery show grade 4 bone formation with tumor cell rimming.](/content/156/2023/1/1/img/IJBI-1-1-6-g0009.png)
- Grade 4 osteoid matrix: [a, b and magnified view (c)] Bilateral CC and MLO mammograms of a 66-year-old woman who had undergone right breast conservation therapy in 2003 and developed a left breast malignancy 15 years later. The mammogram shows postoperative changes in the right breast in the form of minimal architectural distortion and skin retraction in the upper outer quadrant of the right breast, which is also smaller than the left. There is an irregular high-density mass with indistinct margins in the upper central region of the left breast with large coarse calcifications seen within. This patient underwent a lumpectomy. [d, e and magnified view (f)] Bilateral CC and MLO view mammograms (d, e) of a 50-year-old woman, who had undergone right breast conservation therapy in 2014 and developed recurrence in the same breast 7 years later. The mammogram shows the postoperative changes in the right breast with scar site dystrophic calcifications in the lower central region in the retromammary zone (arrows). There is an irregular high-density mass (shouldered arrows) with obscured margins in the upper outer quadrant of the right breast with coarse eccentric calcifications. This patient underwent completion mastectomy. (g, h and i) Histopathological images of the breast masses post-surgery show grade 4 bone formation with tumor cell rimming.

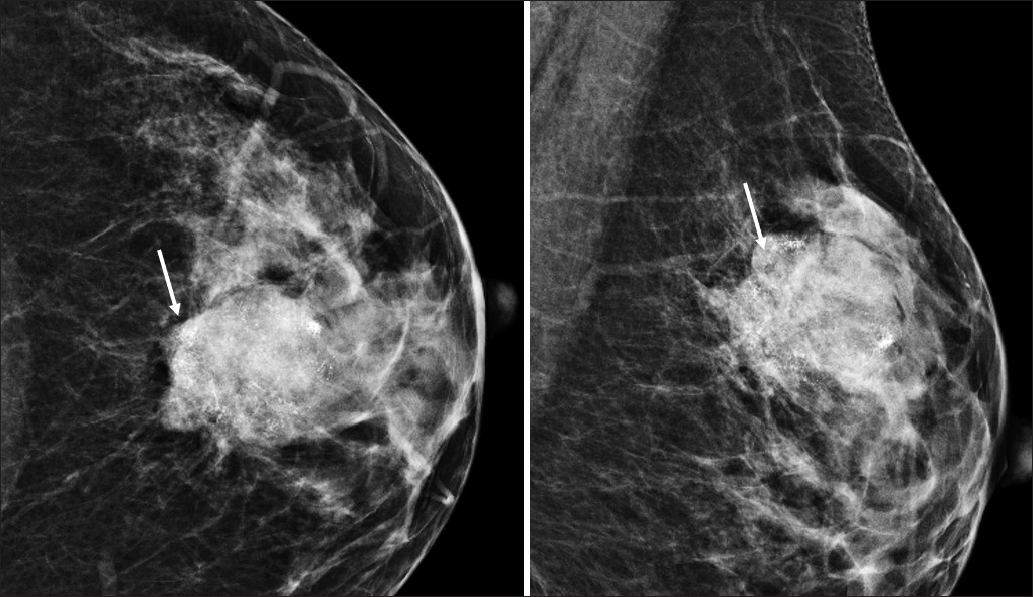
- Grade 1 osteoid matrix: (a, b and c) Bilateral CC and MLO mammograms of a 34-year-old woman shows an oval large high-density mass with obscured margins involving the inner half of the left breast with scanty coarse calcifications within (black arrow). (d, e) Histopathological images of the left breast mass post mastectomy shows grade 1 osteoid matrix.

- Grade 2 osteoid matrix: (a, b and c) Bilateral CC and MLO views with digitally magnified MLO view of left breast of a 48-year-old woman shows an irregular high-density mass with indistinct margins in the upper inner quadrant of the left breast with thin mesh-like calcifications within (black arrow). (d, e and f) Histopathological images of the left breast mass post lumpectomy shows grade 2 osteoid matrix.
Most cases of eccentric coarse calcifications seen on mammograms showed type IV matrix, in turn meaning that these cases had mature bone formation with tumor cell rimming type on histopathology.
Three tumors produced an osteoid matrix in our study, namely, metaplastic carcinoma, primary osteosarcoma, and malignant phyllodes tumor.
Frank bone formation and eccentric coarse calcifications were seen with frank osteosarcoma-like tumors. Cases 2, 3, 4, and 8 in [Table 1] had identical osteosarcoma elements and varied only in the additional finding of malignant epithelium component within the tumor changing the diagnosis to metaplastic carcinoma in the former two.
Malignant phyllodes tumors with mixed chondroid elements produced more hyaline osteoid and less coarse calcification. Occasionally, metaplastic carcinoma produced a similar pattern and less calcified matrix.
To summarize, coarse calcifications mostly indicate metaplastic carcinoma with osteosarcoma element and preoperative diagnosis can guide pathology sampling.
Discussion
The majority of reports describing osteoid-containing lesions focus on case reports of primary osteosarcoma of the breast. Silver et al. presented one of the largest series, comprising 50 cases of primary osteosarcoma of the breast on histopathology.[2] To the best of our knowledge, no prior study has quantitatively correlated osteoid-containing lesions on mammograms with their corresponding histopathology. This study is unique in that we attempted to grade osteoid-containing lesions based on findings from mammography and histopathology.
In this audit, tumors with well-developed focal osteosarcoma elements (Type III) or those composed entirely of fully mature bony trabeculae with tumor cell rimming (Type IV) exhibited larger and coarser calcifications on mammograms. Tumors with Type I and II matrices on histopathology showed no calcification, or displayed thin, mesh-like calcifications, or scanty coarse calcifications on mammograms.
Eccentric coarse calcification can be described as high-density, large calcifications within a specific region of the tumor. This pattern is generally not observed in ductal carcinoma, where calcifications primarily occur within the duct lumens due to calcium deposition in necrotic apoptotic cells.[3] However, in these tumors, calcification or ossification of the matrix occurs in the interstitium and tends to be larger compared to the calcifications seen in ductal carcinomas.
As mentioned earlier, our study identified three tumor types that produced osteoid matrix: metaplastic carcinoma, primary osteosarcoma, and malignant phyllodes tumor.
Metaplastic carcinoma is an uncommon (less than 5% of breast cancer) but aggressive breast cancer variant, exhibiting both epithelial and mesenchymal differentiation.[4,5] It can present in various subtypes, including those that produce a matrix and tend to exhibit calcifications and ossification.[6] Previous literature supports the presence of amorphous and coarse calcifications in cases of metaplastic carcinoma with chondroid/osteoid differentiation.[6,7] These findings align well with our hypothesis regarding the grading of calcifications on mammograms and histopathology. Cases 2, 3, 4, and 8 exemplify the importance of additional sampling to differentiate primary osteosarcoma from metaplastic carcinoma when an osteosarcomatous component is present. Therefore, radiological findings can guide the need for further sampling in such cases. It’s worth noting that many pathology specimen with calcification undergo decalcification while processing sample, which may result in the loss of cellular details and biomarkers for breast cancer.
Malignant phyllodes tumors are known to exhibit heterologous sarcomatous components such as chondrosarcoma or osteosarcoma, leading to the presence of coarse and plaque-like calcifications.[8,9]
Primary sarcoma of the breast is another rare and aggressive form of breast cancer, accounting for less than 1% of all breast cancers, with osteosarcoma comprising approximately 12.5% of cases.[10,11] Osteosarcomatous changes in these cases manifest as coarse-speckled or ivory-like osteoid calcifications.[12]
In our study, we aimed to correlate the amount of ossification/calcifications observed on mammograms with the type of calcified or ossified matrix identified on histopathology. Unlike previous studies, which primarily focused on case reports with limited cases, we attempted to establish this correlation.
Limitations of the study
The major limitations of this study are the small number of cases and its retrospective nature. Since these are rare tumors, it is challenging to study a large number of cases. Despite identifying 25 cases on histopathology, we were able to evaluate only 8 cases as some patients had mammograms performed elsewhere or came to our institution after surgical excision for further medical management.
Ultrasound (US) images for all cases were not available for review, so US findings were not included in the evaluation. This limitation prevents us from commenting on the presence of small satellite lesions, which may be obscured due to dense surrounding parenchyma. In addition, lymph node evaluation based solely on mammograms is incomplete.
As this was a retrospective evaluation conducted over an extended period, the evaluation of immunohistochemical markers varied, making it difficult to assess in this audit.
Conclusion
When eccentric, large, and coarse calcifications are visible within a mass on a mammogram, it raises suspicion of mature bone formation with tumor cell rimming within the tumor matrix.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Infiltrating ductal carcinoma of the breast with osseous metaplasia: imaging findings with pathologic correlation. AJR Am J Roentgenol. 1999;172:1420-2.
- [CrossRef] [PubMed] [Google Scholar]
- Primary osteogenic sarcoma of the breast: a clinicopathologic analysis of 50 cases. Am J Surg Patho. 1998;22:925-33.
- [Google Scholar]
- Calcification in breast lesions: pathologists’ perspective. J Clin Pathol. 2008;61:145-51.
- [CrossRef] [PubMed] [Google Scholar]
- Review of metaplastic carcinoma of the breast: imaging findings and pathologic features. J Clin Imaging Sci. 2012;2:21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Imaging differences in metaplastic and invasive ductal carcinomas of the breast. AJR Am J Roentgenol. 2007;189:1288-93.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging findings of metaplastic carcinoma of the breast with pathologic correlation. J Belge Radiol. 2018;102:46.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Imaging features of metaplastic carcinoma with chondroid differentiation of the breast. AJR Am J Roentgenol. 2007;188:691-6.
- [CrossRef] [PubMed] [Google Scholar]
- Phyllodes tumours of the breast: radiological presentation, management and follow-up. Br J Radiol. 2014;87:20140239.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- AIRP best cases in radiologic-pathologic correlation: malignant phyllodes tumor with osteosarcomatous differentiation. Radiographics. 2013;33:1377-81.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging features of primary breast sarcoma. AJR Am J Roentgenol. 2012;198:W386-93.
- [CrossRef] [PubMed] [Google Scholar]
- Primary osteosarcoma of the breast. Radiographics. 2019;39:626-9.
- [CrossRef] [PubMed] [Google Scholar]








