Translate this page into:
Cavernous hemangioma of breast: A case report

Corresponding author: Varsha Suhas Hardas, Department of Imaging, Grant Medical Foundation and Ruby Hall Clinic, Pune, Maharashtra India.varsha.hardas@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Hardas VS, Mane A, Punya J, Paulraj R. Cavernous hemangioma of breast: A case report. Indian J Breast Imaging 2023;1:31-5.
Abstract
Cavernous hemangioma of the breast is a rare benign vascular tumor, whose clinical diagnosis is challenging. We describe a case of cavernous hemangioma in an 81-year-old female, who presented with a history of a slow-growing lump in the left breast from the past few months. Mammography revealed a circumscribed high-density mass with microlobulated margins in the lower outer quadrant with no associated findings. Targeted sonography revealed an isoechoic heterogeneous solid mass in the subcutaneous plane with no associated vascularity on color doppler. It was assigned as BI-RADS IV B and an image-guided core biopsy was done. The histopathology report was of cavernous hemangioma. A close follow-up was advised.
Keywords
Breast vascular tumor
cavernous hemangioma
benign breast tumor
CASE PRESENTATION
An 81-year-old female presented to the breast imaging unit with complaints of a lump in the left breast for the past few months. There was no history of associated pain, skin discoloration, change in breast size, or similar lumps elsewhere in the body. She attained menarche at the age of 13 and menopause at 54, and there was no history of use of oral contraceptive pills. There was no family history of breast or ovarian malignancies.
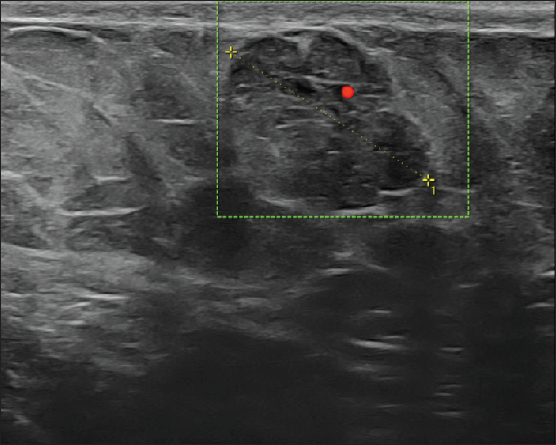
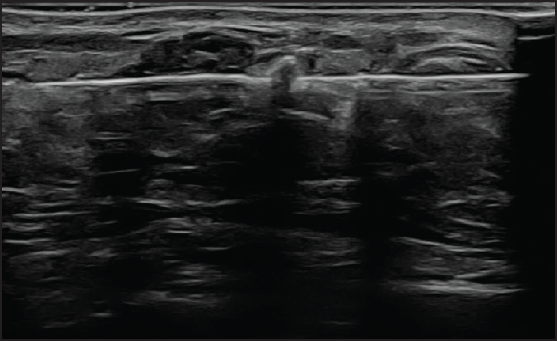
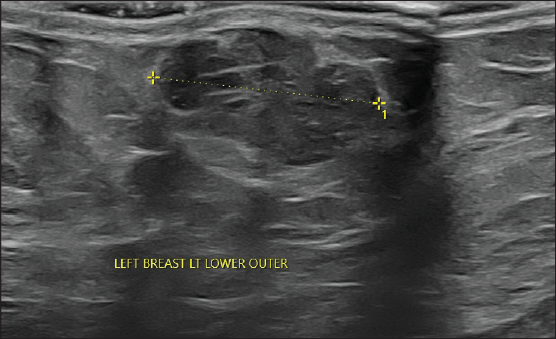
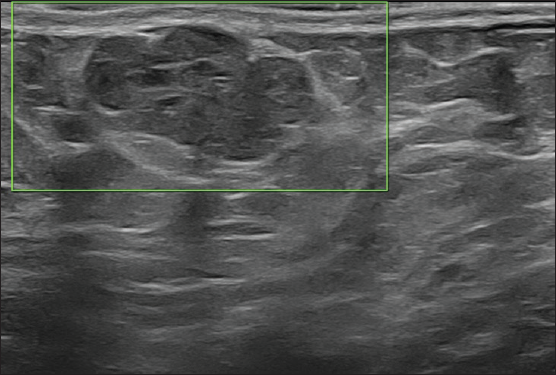
On local examination, a small mobile firm mass was found in the lower outer quadrant of the left breast. There was no associated skin discoloration. A digital mammogram with 3D tomosynthesis of both breasts was performed. It showed a circumscribed high-density mass with microlobulated margins in the lower outer quadrant of the left breast [Figures 1a and 1b] with no associated microcalcifications. There was no other focal lesion or associated skin thickening, nipple retraction, or axillary lymphadenopathy. This was followed by targeted breast ultrasound, which showed heterogeneously solid mass (21 × 11 × 21 in mm size) in the left lower outer quadrant in the subcutaneous plane, parallel in orientation, isoechoic to adjacent subcutaneous fat [Figure 2]. No other focal lesions were seen in either breast. No significant vascularity was detected on Color Doppler [Figures 3 and 4]. The lesion was considered BI-RADS 4 b and a Ultrasound-guided core needle biopsy was performed using a 16 gauge gun (5 cores) [Figure 5]. Histopathology showed dilated blood vessels with intravascular red blood cells (RBCs) with compressed endothelial cells separated by fibrous stroma [Figures 6, 7 and 8]. There was no evidence of atypia or malignancy in the sections studied. Keeping in mind the clinical, radiological, and pathological findings of a benign lesion, the patient was advised for a follow-up and to visit the hospital in case of a sudden increase in the size of the lump. On 6 months ultrasound follow-up, there were no interval changes in lesion size or morphology [Figures 9 and 10].
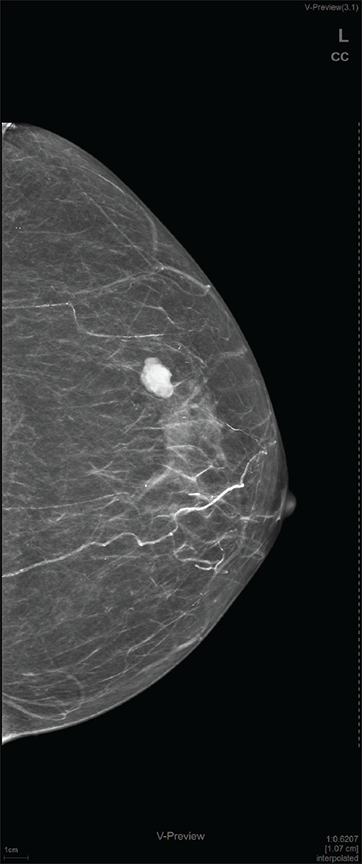
- Craniocaudal view mammogram of the left breast showing fairly well-circumscribed high-density mass with microlobulated margins in the lower outer quadrant.
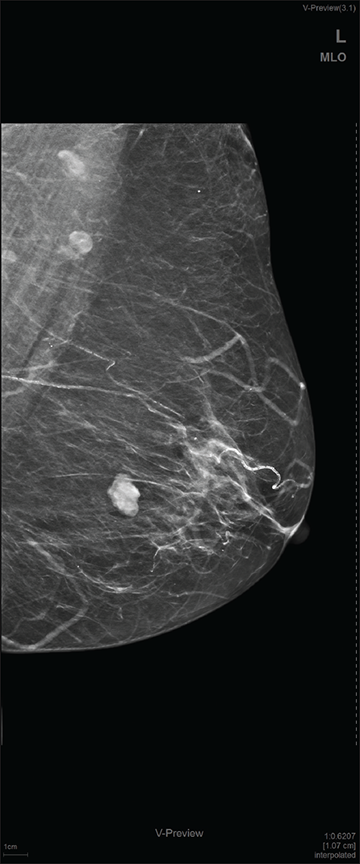
- Mediolateral oblique view mammogram of the left breast showing fairly well-circumscribed high-density mass with microlobulated margins in the lower outer quadrant.
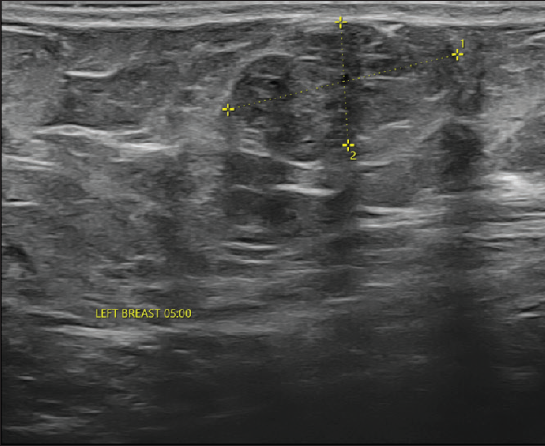
- On targeted ultrasound isoechoic heterogeneously, solid mass of size 21 × 11 × 21 mm is noted.
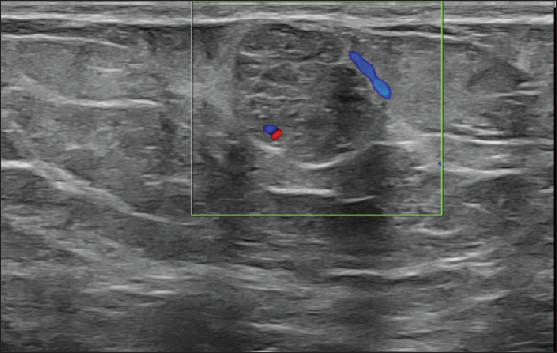
- Color doppler image showing minimal internal and peripheral vascularity.
- Color doppler image showing minimal internal vascularity.
- Ultrasound-guided core needle biopsy was performed.
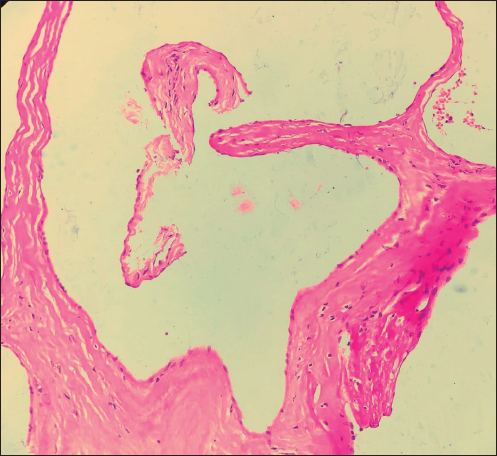
- 40×: Section shows dilated blood vessels lined by benign endothelial cells.
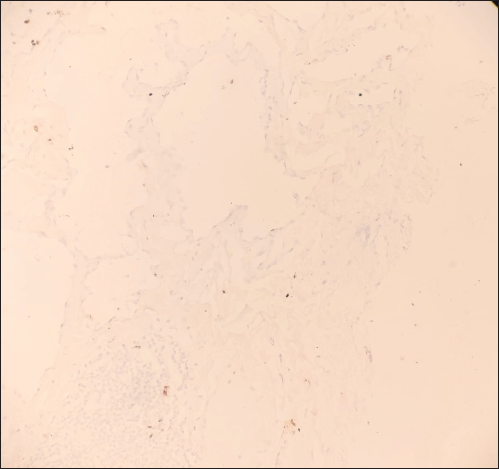
- 10×: Lesional cells are immunonegative for CK suggesting nonepithelial origin.
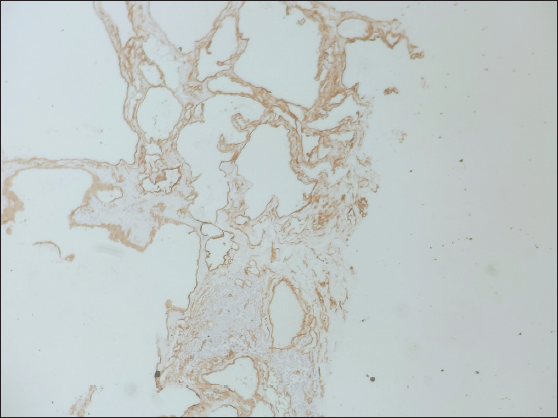
- Lesional cells show immunoreactivity for CD34 suggesting endothelial cell origin of dilated tissue.
- On 6-month follow-up, ultrasound was predominantly unchanged showing isoechoic heterogeneously solid mass measuring 20 × 10 mm.
- On 6-month follow-up, ultrasound revealed no demonstrable vascularity.
DISCUSSION
Hemangiomas of the breast are uncommon benign vascular tumors and represent only 0.4% of all breast tumors.[1] Common vascular lesions of the breast are hemangiomas, lymphangiomas, and angiolipomas. Hemangiomas are classified as cavernous, venous, or capillary. Cavernous are the most common subtype, originating from mature blood vessels.[2]
Clinically, cavernous hemangioma patients present with a lump in the breast or skin discoloration. Most commonly, hemangiomas are located in the sub-dermal or subcutaneous plane.[3]
On mammography, hemangiomas can appear as a well-circumscribed mass with microlobulated margins in the superficial plane and not in the parenchymal zone and may have calcifications due to phleboliths.[2,3] On ultrasound, they usually present as circumscribed, oval/round, hypo-/isoechoic superficial lesions. It can be difficult to differentiate them from the surrounding tissue as they are mostly isoechoic masses. Vascularity on color doppler sonography does not have any diagnostic significance, as they may present as hypo or hypervascular lesions.[4,5]
Since hemangiomas do not have any distinct imaging features and it is difficult to differentiate from angiosarcoma and mucinous lesions, histopathology is essential to establish a definitive diagnosis. Histopathology usually shows benign endothelial-lined dilated vascular channels filled with red blood cells which show strong immunoreactivity for vascular markers like CD34 [Figures 6, 7 and 8]. Cellular atypia and/or mitosis are not seen.[2,6]
Hemangiomas can enlarge over time.[1] Estrogen influence has been described in literature, in the enlargement of hemangiomas, also suggesting its possible role in the development of these lesions.[7]
Hemangiomas have a good prognosis when excised completely.[8] However, surgical excision for hemangiomas can be avoided if there is no atypia on core biopsy, and complete surgical resection is advised for those lesions with atypical findings on histology to rule out the possibility of an angiosarcoma. Recent literature suggests that benign hemangiomas can be safely monitored for growth with imaging.[7,9]
CONCLUSION
Breast hemangioma is a rare benign vascular tumor. Although there are no specific diagnostic imaging features, cavernous hemangioma should be considered in the differential diagnosis when an oval or round, iso-high density, circumscribed mass is found in a superficial location on mammography and circumscribed, iso-/hypoechoic lesion with lobulated margins is seen on the USG in the subcutaneous plane. The presence of phleboliths can clinch the diagnosis when present. Histopathology confirmation is always recommended, as the imaging features are otherwise nonspecific. If histology shows no atypia, they can be safely followed up on imaging.
Acknowledgement
The author would like to thank Dr. Ankitha Tamhane from the Department of Pathology for providing the histology slides.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Sinusoidal hemangioma of the breast: diagnostic evaluation management and literature review. Gland Surg. 2017;6:105.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cavernous hemangioma of the breast: Radiologic and pathologic findings. Breast J. 2019;26:531-3.
- [CrossRef] [PubMed] [Google Scholar]
- Cavernous hemangioma in a male breast: A rare entity and overview of current literature. Radiol Case Rep. 2020;15:1386-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mammographic and sonographic findings of a breast subcutaneous hemangioma. J Ultrasound Med. 2002;21:585-8.
- [CrossRef] [PubMed] [Google Scholar]
- Breast hemangioma with difficulty in preoperative diagnosis: a case report. World J Surg Oncol. 2014;12:1-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Benign vascular lesions of the breast diagnosed by core needle biopsy do not require excision. Histopathol. 2017;71:795-804.
- [Google Scholar]
- A cavernous hemangioma located in the axillary area: Challenges in preoperative diagnosis and operation. Korean J Clin Oncol. 2019;15:127-31.
- [Google Scholar]
- The blue alert: an unusual case report of breast hemangioma. Egypt J Radiol Nucl Med. 2022;53:221.
- [Google Scholar]








