Translate this page into:
Role of empirical antitubercular therapy for chronic mastitis—A prospective study
*Corresponding author: Smriti Hari, Department of Radiodiagnosis and Interventional Radiology, All India Institute of Medical Sciences, New Delhi, Delhi, India. drsmritihari@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Baby A, Hari S, Paul SB, Manchanda S, Kataria K, Mathur S, et al. Role of empirical antitubercular therapy for chronic mastitis—A prospective study. Indian J Breast Imaging. 2024;2:13-20. doi: 10.25259/IJBI_2_2024
Abstract
Objectives
Chronic mastitis (CM) is an uncommon inflammatory condition of the breast, clinically mimicking malignancy with variable etiological factors. It often recurs, causing cosmetic disfigurement and hampering the quality of life of affected females. Limited literature reveals underlying bacterial infection as causative agents in a few cases, while the majority belong to idiopathic entities. CM can be seen as two histological types—chronic granulomatous mastitis (CGM) and chronic nonspecific inflammatory infiltrates (NGM). Etiological and ethnic variations exist globally, and there is lack of information from India. Understanding this entity is important for undertaking etiology-based therapy and preventing unnecessary mismanagement.
Materials and Methods
We evaluated the demographic, imaging profile and management protocol of CM patients presenting to a tertiary care center in India. Clinically suspected CM patients were prospectively evaluated by mammography and ultrasound (USG). USG-guided biopsy was performed for histopathological and microbiological analysis and treatment was administered accordingly. Response assessment was conducted at six months through USG and clinical evaluation.
Results
Out of the 53 clinically suspected CM patients, 48 biopsy-proven CM patients were enrolled (five breast carcinoma cases were excluded). All 48 patients were females, with a mean age of 36 ± 9 years. The most common symptom was a unilateral painful lump with skin discoloration. The etiology was idiopathic in 58.3% of cases, tuberculosis in 29%, and bacterial in 4%. Mammography revealed focal asymmetry, architectural distortion, and trabecular thickening. USG showed tracking fluid collections, microlobulated hypoechoic masses, and skin thickening. Etiology-based treatment was administered, and all patients showed 90–100% resolution between four and six months.
Conclusion
Mammography and USG are important for raising the suspicion while histopathology and microbiological analysis are mandatory for accurate treatment of CM. In India, idiopathic and tubercular etiology of CM is encountered and patients respond well to therapy.
Keywords
Chronic granulomatous mastitis
Tubercular mastitis
Etiology
Mammography
Ultrasound
Therapeutic response
INTRODUCTION
Chronic Mastitis (CM) is a benign inflammatory condition of the breast. Patients often present with inflammatory symptoms like nipple retraction/discharge, skin retraction, sinus tracts, and skin ulcers.[1] These features mimic breast carcinoma, making the diagnosis and management very challenging.
There are multiple etiologies of CM, and majority of the published studies are from the developed world[2–5] focusing mainly on idiopathic granulomatous mastitis (IGM). Additionally, ethnic differences are also prevalent.[2,6,7] While IGM and autoimmune etiologies are reported from the West, few anecdotal reports from developing countries like ours have documented cases of tubercular mastitis (TM).[7,8] Diagnosis of TM is challenging as the positivity of acid-fast bacillus (AFB) is quite infrequent, and thus microbiological examination with specialized tests for tuberculosis (TB) may have a role in specific diagnosis.[9] Histology is mandatory for a definitive diagnosis.
Mastalgia hampers clinical evaluation and the role of imaging becomes extremely vital. Familiarity with the variable imaging spectrum of CM is important for treating surgeons, in order to provide accurate diagnosis and relieve the anxiety of the patient, thereby avoiding mismanagement. Mammography and ultrasound (USG) are the imaging modalities used for evaluating these patients. Considering the prevalence of TB in variable forms in our country, such patients are often put on empirical antitubercular therapy (ATT). However, there is limited literature in the form of case series and case reports to support the evidence of tubercular treatment in CM patients. It thus remains dubious which subset of females should receive ATT and which should not. Hence, this prospective study was planned to evaluate the etiopathogenesis, imaging profile, outcome of the microbiological assessment, and therapeutic outcome of patients of CM presenting to a tertiary care center in India.
MATERIALS AND METHODS
Patients with clinical suspicion of CM (breast pain and redness with palpable lump or discharging sinus tracts) presenting to the surgery outpatient were included in this study after obtaining approval from the Institute’s Ethics Committee and informed written consent from each patient. Lactating mothers and biopsy-proven carcinoma breast cases were excluded.
Clinical and imaging evaluation
History of risk factors like parity, breast feeding, menstrual history, oral contraceptive intake, hormonal replacement therapy, diabetes, TB, and autoimmune disease was obtained and clinical breast examination was done. Mammography was done in patients above 30 years according to American College of Radiology (ACR) Appropriateness Criteria.[10] Craniocaudal (CC) and mediolateral oblique (MLO) views along with tomosynthesis were obtained on Hologic Selenia Dimensions full-field digital mammography system. After reviewing the mammography images, all patients underwent B mode USG of the breast by either of the coinvestigators (experience ranging from 2 to 15 years) using Aixplorer USG system on the same day (SuperSonic Imagine, Aix-en Provence, France). Patient lying supine and ipsilateral arm above the head, the entire affected breast was screened by 4–15 MHz linear array transducer, taking overlapping scans in transverse and radial planes from the periphery to the nipple, ensuring complete coverage of the breast, axillae, and the contralateral normal breast. Imaging interpretation was done by two investigators according to the fifth edition of the ACR breast imaging reporting and data system (ACR-BIRADS).
Histopathology and microbiology
Under aseptic conditions, USG-guided core needle biopsy of the breast mass was performed within one week of mammogram. Six cores of tissue were obtained—three cores for histopathological analysis in formalin solution and hematoxylin–eosin (HE) staining while three cores for microbiological analysis (bacteriology, TB, and mycology) in normal saline. Gram stain, conventional culture, and further characterization with MALDI-TOF mass spectrometry was done in bacteriology. Ziehl Neelson staining, polymerase chain reaction (PCR), Gene Xpert, and liquid culture were done for TB bacilli. KOH (potassium hydroxide) smear and culture was done for fungal elements.
Treatment and follow up
Treatment was based on the etiology and the symptoms as per clinical assessment. USG and the clinical examination were repeated at four to six months. A scoring system comprising of the clinical and imaging criterion on a scale of –1 to 2 was developed (the score obtained for each was multiplied by a factor of 10 to calculate the percentage resolution or worsening).
Definitions
Suspicious symptoms of CM were breast pain, redness, lump, or discharging sinus tracts.
Complete disappearance of mass, tracking fluid collection, and edema on USG and complete absence of pain and redness of skin on follow up was termed complete resolution. Partial resolution was the reduction in the USG findings and clinical symptoms. No change in USG and clinical features was considered stable disease. Increase in mass size, edema, and tracking fluid collection and clinical features of increased pain and skin redness, without an intervening period of improvement, was taken as worsening. Relapse was considered as a new disease process and was not included in the scoring system.
Points assigned were—complete resolution: 2 points, partial resolution: 1 point, stable disease 0 point, and worsening: –1 point. Total points (out of 10) for each patient were calculated and multiplied by a factor of 10 to obtain the percentage resolution or worsening.
Data collection and statistical analysis
SPSS (version 23) was used. Continuous data were expressed as mean and standard deviation (SD) and categorical data as proportions. Comparisons of the clinical, demographical profile, and imaging parameters of different types of CM were done using chi-square and Fisher’s exact test. Value of significance (p-value) was calculated using the Mann Whitney test in case of skewed data and p-value of less than 0.05 was considered significant.
RESULTS
Fifty-three clinically suspected CM patients were evaluated. Five patients had breast malignancy and were excluded. The remaining 48-proven CM patients were enrolled. Common presenting symptoms were painful lump, discharge from multiple skin sites, skin erythema, and sinus tracts [Figure 1]. Detailed profile of patients is depicted in Table 1.
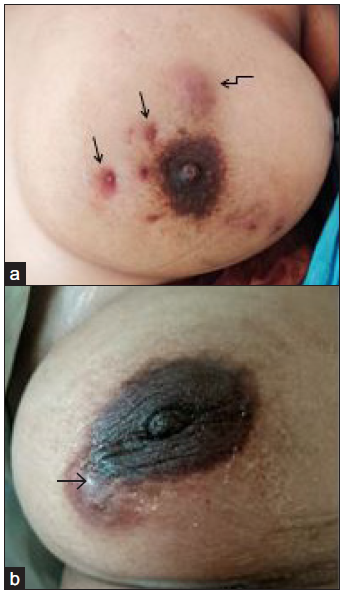
- Clinical photographs of chronic mastitis (CM) patients (a) left breast of a 36-year-old female showing skin erythema (curved black arrow), multiple sinus tracts (black arrows) and discharge from multiple skin site (b) skin erythema (black arrow) in the right breast of a 32-year-old female.
| Variable | Number/Value |
|---|---|
| Age (years); Mean (SD) | 35.7 (9.0) |
| Breast involved | n (%) |
| Right | 25/48 (52.1%) |
| Left | 20/48 (41.7%) |
| Bilateral | 3/48 (6.2%) |
| Menstrual status | n (%) |
| Premenopausal | 44/48 (91.7%) |
| Postmenopausal | 4/48 (8.3%) |
| Significant history | n (%) |
| Breast feeding | 43/48 (89.5%) |
| Diabetes | 2/48 (4.2%) |
| Menstrual abnormalities | 4/48 (8.3%) |
| OCP intake | 3/48 (6.3%) |
| TB/TB contacts | 0 |
| Autoimmune disease | 0 |
| Presenting symptoms | |
| Lump | 48/48 (100%) |
| Pain | 45/48 (93.7%) |
| Skin discoloration | 44/48 (91.6%) |
| Nipple discharge | 4/48 (8.3%) |
| Nipple retraction | 4/48 (8.3%) |
| Sinus opening in skin | 10/48 (20.8%) |
| Duration of symptoms (months), mean (SD) | 6.4 (3.3) |
| Imaging spectrum | |
| Ultrasound (n = 48) | |
| Irregular hypoechoic mass with posterior enhancement | 29/48 (60%) |
| Circumscribed hypoechoic mass/abscesses | 10/48 (21%) |
| Architectural distortion | 48/48 (100%) |
| Increased vascularity | 44/48 (92%) |
| Tracking fluid collection | 41/48 (85%) |
| Skin thickening | 35/48 (73%) |
| Parenchymal edema | 46/48 (96%) |
| Axillary lymph nodes | 26/48 (54%) |
| Mammography (n = 28) | |
| Focal mass | 9/28 (32%) |
| Architectural distortion | 19/28 (68%) |
| Focal asymmetry | 14/28 (50%) |
| Calcifications | 2/28 (7%) |
| Axillary lymph nodes | 20/28 (71%) |
| Nipple retraction | 3/28 (11%) |
| Skin thickening | 11/28 (39%) |
| Trabecular thickening | 18/28 (64%) |
SD: Standard Deviation, OCP: Oral contraceptive pills, TB: Tuberculosis.
All patients underwent USG while mammography was performed in 28/48 (58%). No patient had active or old pulmonary TB on chest radiograph. Mammography depicted architectural distortion 19/28 (68%), trabecular thickening 18/28 (64%), focal asymmetry14/28 (50%), and a breast mass in 9/28 (32%) patients [Figure 2]. Axillary lymphadenopathy was present in 20/28 (71%).
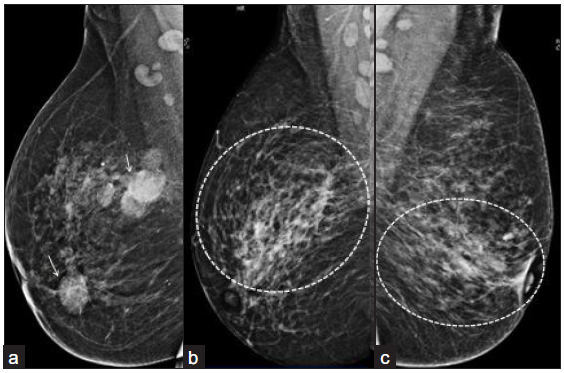
- (a) Right breast mammogram MLO view of a 38-year-old female showing, multiple circumscribed, high density masses in upper central and inner quadrant (white arrows) with scattered, benign, calcific foci and axillary lymph nodes. (b) Right breast mammogram (MLO view of another 36-year-old female showing focal asymmetry (outer and central quadrant) with surrounding trabecular thickening (dotted circular area). Left breast mammogram MLO view (c) of a 33-year-old female showing architectural distortion and trabecular thickening (dotted circular area). MLO: Mediolateral oblique.
On USG, all had architectural distortion. An irregular, hypoechoic and microlobulated mass was present in 39/48 (81%). Tracking fluid collections with increased vascularity was the hallmark finding in 41/48 (85%). Posterior acoustic enhancement was seen in 38/48 (97%) patients. A large proportion had inflammatory features of skin thickening 35/48 (73%) and edema (46/48, 96%) [Figure 3].
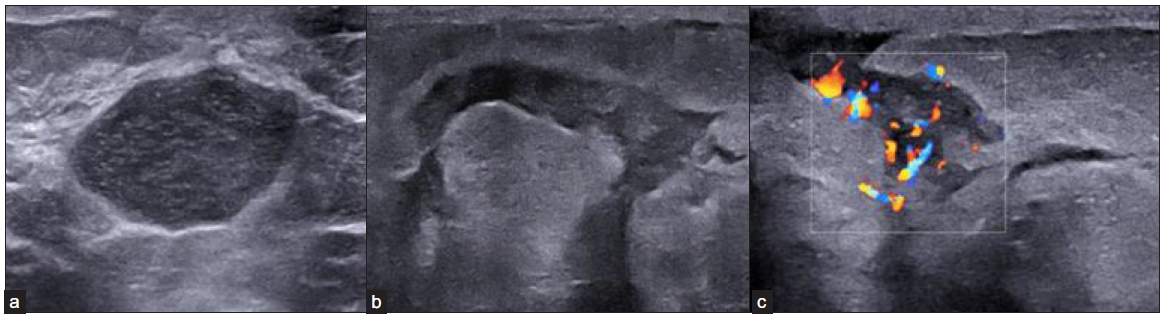
- (a) Left breast USG image of a 30-year-old female, (b) Showing a well-defined, cystic lesion with thick internal echoes and posterior enhancement suggesting formed abscess. Left breast USG of a 31-year-old female, (c) Showing tracking fluid channels with increased vascularity on color Doppler. USG: Ultrasonography.
Two histological types of CM were diagnosed on biopsy—CGM 30/48 (62%) and nongranulomatous mastitis (NGM) 18/48 (37%) [Figure 4]. CGM showed moderate to severe lobulocentric, noncaseating granulomatous inflammation with or without necrosis. The granulomas comprised of collections of neutrophils, lymphocytes, plasma cells, epithelioid cells, and giant cells. NGM type depicted chronic nonspecific inflammatory infiltrates without any granulomas. One patient had duct ectasia along with chronic inflammation. Caseating necrosis, a classical feature of TB, was not seen in any patient.
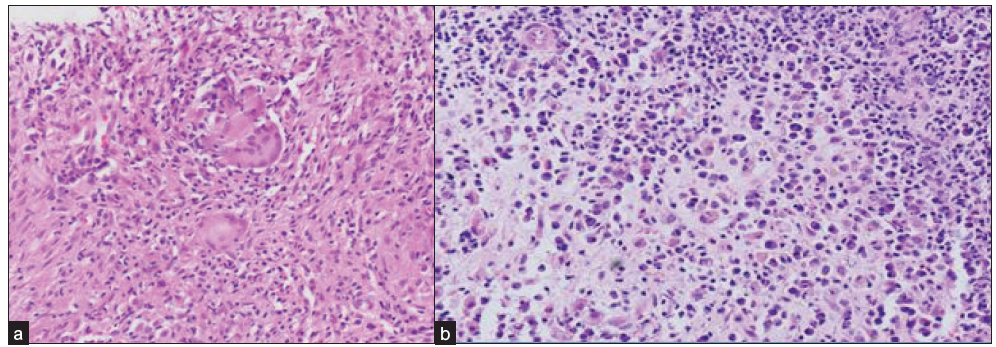
- Photomicrographs of breast biopsy (H&E stain, 200x) shows (a) epithelioid cell granulomas with multinucleated giant cells suggestive of CGM, (b) dense lympho-plasmacytic infiltrates without any well-formed granulomas diagnostic of NGM. H&E: Hematoxylin and eosin, CGM: Chronic granulomatous mastitis, NGM: Nongranulomatous mastitis.
Bacterial colonies were detected in 2/48 (4.1%) patients (one Staphylococus warneri and Acinetobacter baumanii, the other methicillin-resistant staphylococcus aureus [MRSA]). No patient had any fungal elements in KOH smear and fungal culture. Only one patient was positive on Gene Xpert and culture for TB. Fourteen patients (29%) had clinical suspicion of TB, CGM on histology but no microbiological evidence of TB. In the remaining 32/48 (67%) patients, no underlying etiology was established and were labeled IGM. Patients with clinical suspicion of TB exhibited symptoms such as skin erythema and pain similar to other patients. However, sinus tracts and discharge from multiple sites were frequently observed. Large abscesses on imaging were also seen commonly in those patients [Figure 5].
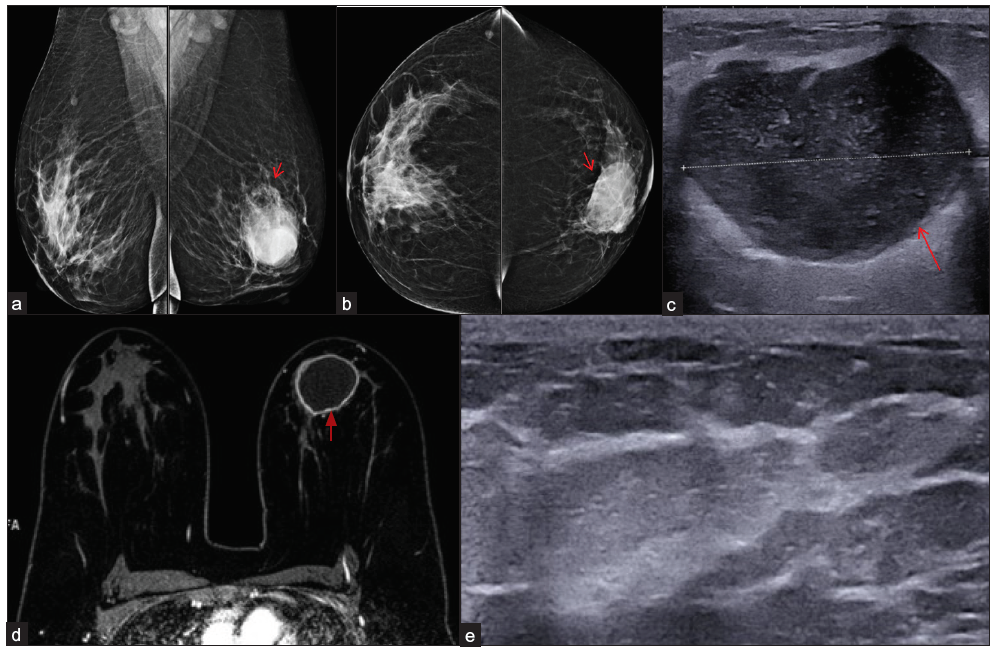
- Tubercular mastitis (Gene Xpert and culture positive) in a 38-year-old lady, bilateral mammogram mediolateral oblique (a) and cranio-caudal (b) views showing an oval circumscribed high density mass involving left breast lower inner quadrant (red arrows). B-mode ultrasound image (c) showing oval hypoechoic circumscribed mass (red arrow) with posterior acoustuic enhancement and moving internal echoes s/o abscess. The horizontal white line through the abscess represents its diameter. (d) Dynamic contrast enhanced -MRI image shows a large abscess (red arrow). B-mode ultrasound image (e) at six months of Anti-tubercular therapy shows complete resolution of the abscess.
All patients except one had received single or multiple courses of antibiotics prior to reporting to us. Fourteen histology-proven CGM patients with clinical suspicion of TB were given antitubercular therapy (ATT) after two weeks when they showed no response to antibiotics. ATT was given for a period of six months and no significant side effects were observed during the therapy period. Abscess drainage was performed in 22/48 (46%) patients. Five patients (10%) after completion of family underwent duct excision and steroids were given to two patients (4%). One patient refused treatment after knowing the benign nature of the disease but continued to be under observation.
All patients developed clinical and radiological improvement at four to six months. Thirty five (73%) patients had 90–100% resolution and 13/48 (27%) had 70–80% resolution of the disease. None of the patients developed recurrence during the entire two years study period.
DISCUSSION
About 92% of the CM patients in our study were in the reproductive age group (31–40 years), quite similar to the reported literature.[4,11] This is perhaps due to the higher mammary glandular activity at this age. However, CM is also reported in extreme age groups as well.[1,12] Although there is no known association of CM with breastfeeding, it is often seen in breastfeeding females.[11,13,14] We had 43/48 (89%) patients with breastfeeding history, of which 72% developed CM within five years of breastfeeding. Nonetheless, four nulliparous females also suffered from this disease. Since CM also occurs in males,[15] thus additional etiological factors could also have a role.
The inflammatory symptoms are often mistaken for inflammatory breast cancer (IBC) and often misdiagnosed.[16] Erythema occupying at least one-third of the breast, edema, and/or peau d’orange appearance, warm breast with or without underlying palpable mass are the common manifestations of IBC. This presentation could be misinterpreted for any bacterial infection or a breast abscess. However, IBC is acute, often occurring in less than three months’ duration. In contrast, invasive breast carcinoma often presents as a painless lump or nipple discharge and may have secondary features of skin thickening/retraction or skin ulceration with fungating lesion in the advanced stages.[13,17,18] The axillary lymph nodes are more commonly involved. Ductal carcinoma in situ patients are usually asymptomatic and are detected on screening mammogram.[19] Thus, imaging-based histopathology and microbiology is crucial.
Multiple hypotheses like autoimmune and infectious causes have been proposed on the etiopathogenesis of CM with little evidence.[2,7,9] Corynebacterium has been the most common causative agent.[20,21] Not much data is available from the Asian population.[12,22,23] We found no causative organism in 45/48 (93.7%) of our patients on microbiological analysis and just two patients showed bacterial growth on culture. It was possibly due to the antibiotic treatment given to them due to their inflammatory symptoms prior to reporting to our center.
Sinus formation in 14–18% and axillary lymphadenopathy in 28–61.5% has been reported in CGM,[15] whereas these are uncommonly present in IGM.[1] Since TB is endemic in India, we found discharging sinus tracts from multiple skin sites in 10/48 (21%), skin erythema in 44/48 (92%), nipple discharge in 5/48 (10%), and frequent lymph node involvement and sinus tract formation. A study from Northern India documented discharging sinuses in 15.7%, palpable breast lump in 31.5%, and axillary lymphadenopathy in as high as 47.3% of TM patients.[7]
Prevalence of TM in India is 4.1%.[7,9] A plethora of tests have been recommended and AFB identification on microbiological analysis is the gold standard. Since the microbiological yield in the breast tissue is just 25%, diagnosis of TB is challenging.[7] We had 14/48 (29%) patients with clinical suspicion of TB and histopathological evidence of granulomas, but only one case was confirmed on gene Xpert and culture. These 14 patients had not responded to prior antibiotic therapy and improved with ATT by six months. Such patients have been considered as index cases of TB in certain other studies as well.[7,9,24,25] Thus, it is important to consider TM as an important etiology in situations where the clinical suspicion is high and there is no response on routine antibiotics. This would undeniably avoid delays in treatment and facilitate patient recovery.
The mammography features of focal asymmetry, architectural distortion, trabecular thickening, and, in some cases an oval circumscribed mass appearing as an abscess on USG should raise the suspicion of CM. Such patients merit further histopathological confirmation. Other studies have also reported similar findings.[4,8,15] The hallmark feature on USG is the presence of large tracking fluid collections[4,26] which was present in 41/48 (85%) of our patients. This finding facilitates differentiation from malignancy where the surrounding structures are infiltrated and there is a desmoplastic reaction around the invasive component of the mass.
Although uncommon, abscesses are known in advanced cases of CGM.[1] Since we had TM patients, 18/48 (37 %) of them had breast abscesses. Two other Indian studies have reported abscesses in as high as 26–27% of TM patients.[7,9]
Due to lack of existent guidelines on management of CM, treatment is controversial and common approach is to target the etiology and relieve clinical symptoms.[27–29] With the conservative approach, complete regression in 50% cases at 2–24 months has been shown.[12] More interestingly, even spontaneous burning out of IGM after 6–12 months is known.[30–32] We treated patients on the basis of their clinical manifestation and histopathology. Irrespective of the therapy, all patients improved both clinically and radiologically by six months, and no recurrence was noted during the two years of study period. One NGM patient had 100% resolution at six months without therapy.
The study had an important limitation. All patients except one had received antibiotics prior to reporting to us. Thus, the natural history of the disease and causative organism demonstration could not be done in most cases. However, we found that the patients who had not responded to antibiotics could respond to empirical ATT in our population, even in the absence of microbiological evidence of TB. Thus, it could be hypothesized that if conservative management with or without antibiotic therapy seems to be not working for these patients; empirical ATT may be resorted to, especially in patients with breast abscess or sinus formation.
CONCLUSION
Mammography and USG are crucial for diagnosis, accurate sampling, and follow up of CM patients. Histopathology and microbiological analysis are mandatory for guiding therapy. Infective and idiopathic etiology of CM is encountered in the Indian population and patients respond well to treatment. Randomized controlled trials with larger sample size are needed on patients receiving different types of treatment versus no treatment for providing answers to the treatment approach.
Ethical approval
The research/study approved by the Institutional Review Board at All India Institute of Medical Sciences New Delhi, number IECPG-503/29.11.2017-RT 6/20-12-2017, dated 20-12-2017.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Idiopathic granulomatous mastitis: Manifestations at multimodality imaging and pitfalls. Radiographics. 2018;38:330-56.
- [CrossRef] [PubMed] [Google Scholar]
- Aetiology of idiopathic granulomatous mastitis. World J Clin Cases. 2014;12:852-58.
- [CrossRef] [Google Scholar]
- Chronic granulomatous mastitis: A therapeutic dilemma revisited. Arch Int Surg. 2016;6:100-4.
- [CrossRef] [Google Scholar]
- Imaging findings in patients with granulomatous mastitis. Iran J Radiol. 2016;13:e33900.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Red flags for the differential diagnosis of granulomatous mastitis: A case report. J Med Case Reports. 2020;14:215.
- [CrossRef] [Google Scholar]
- Idiopathic granulomatous mastitis: presentation, investigation and management. Future Oncol. 2016;12:1381-94.
- [CrossRef] [PubMed] [Google Scholar]
- Tubercular mastitis: An institutional experience from a tertiary care centre of northern India. Asian J Med Sci. 2017;8:72-5.
- [CrossRef] [Google Scholar]
- Idiopathic granulomatous mastitis: Imaging update and review. Insights Imaging. 2016;7:531-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tuberculous mastitis: Not an infrequent malady. Ann Niger Med. 2011;5:20-3.
- [CrossRef] [Google Scholar]
- Expert panel on breast imaging: ACR appropriateness criteria breast pain. J Am Coll Radiol. 2018;15:S276-82.
- [CrossRef] [PubMed] [Google Scholar]
- Granulomatous mastitis: Etiology, imaging, pathology, treatment, and clinical findings. Breast Cancer Res Treat. 2018;171:527-34.
- [CrossRef] [PubMed] [Google Scholar]
- The role of conservative treatment in idiopathic granulomatous mastitis. Breast J. 2005;11:454-6.
- [CrossRef] [PubMed] [Google Scholar]
- Metastatic breast cancer: Prognosis, diagnosis and oncological management. In: Wyld L, Markopoulos C, Leidenius M, SenkusKonefka E, eds. breast cancer management for surgeons. Cham: Springer International Publishing; 2018. p. :579-94.
- [Google Scholar]
- Granulomatous mastitis: Radiological findings. Acta Radiol. 2007;48:150-5.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic granulomatous mastitis: imaging, pathology and management. Eur J Radiol. 2013;82:165-75.
- [CrossRef] [PubMed] [Google Scholar]
- Inflammatory breast cancer: What we know and what we need to learn. Oncologist. 2012;17:891-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Differentiating inflammatory breast cancer from acute mastitis. Am Fam Physician. 1995;52:929-34.
- [PubMed] [Google Scholar]
- Clinical presentation, diagnosis and staging of breast cancer. In: Breast Cancer Management for surgeons. 2017. p. :159-76.
- [Google Scholar]
- Ductal carcinoma in situ of the breast: Morphological and molecular features implicated in progression. Biosci Rep. 2014;34:e00090.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Idiopathic granulomatous mastitis associated with corynebacterium sp. Infection. Hawaii Med J. 2011;70:99-101.
- [Google Scholar]
- Clinical metagenomic analysis of bacterial communities in breast abscesses of granulomatous mastitis. Int J Infect Dis. 2016;53:30-3.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic granulomatous mastitis: An autoimmune disease? The Scientific World J. 2013;4:148727.
- [CrossRef] [Google Scholar]
- Granulomatous mastitis: Clinical, pathological features, and management. Breast J. 2010;16:176-82.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging features and diagnosis of tuberculosis of the breast. Clinical Radiology. 2017;72:217-22.
- [CrossRef] [PubMed] [Google Scholar]
- Breast tuberculosis in East London: A 13‐year retrospective observational study. Breast J. 2020;26:235-9.
- [CrossRef] [PubMed] [Google Scholar]
- Granulomatous lobular mastitis: Imaging, diagnosis, and treatment. Am J Roentgenol. 2009;193:574-81.
- [CrossRef] [Google Scholar]
- Idiopathic granulomatous mastitis: A medical or surgical disease of the breast? ANZ J Surg. 2015;85:979-82.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment for and clinical characteristics of granulomatous mastitis. Obstet Gynecol. 2015;125:801-7.
- [CrossRef] [PubMed] [Google Scholar]
- Corticosteroid treatment and timing of surgery in idiopathic granulomatous mastitis confusing with breast carcinoma. Breast Cancer Res Treat. 2010;123:447-52.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic granulomatous mastitis: A 25-year experience. J Am CollSurg. 2008;206:269-73.
- [CrossRef] [Google Scholar]
- Management of granulomatous lobular mastitis: An international multidisciplinary consensus (2021 edition) Military Med Res. 2022;9:20.
- [CrossRef] [Google Scholar]
- Idiopathic granulomatous mastitis. Ann Breast Surg. 2020;4 doi:10.21037/abs-20-89
- [CrossRef] [Google Scholar]







