Translate this page into:
Rare breast cancers with seemingly benign features on conventional imaging: Radio-pathological correlation and review of literature

*Corresponding author: Rashmi Sudhir, Department of Radio-diagnosis, Apollo Health City, Hyderabad, Telangana, India. rashmi4210@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sudhir R, Kodandapani S. Rare breast cancers with seemingly benign features on conventional imaging: Radio-pathological correlation and review of literature. Indian J Breast Imaging 2023;1:36-47.
Abstract
Oval or rounded breast masses with circumscribed margins, absence of microcalcifications, and architectural distortions are usually benign. However, 10-20% of breast cancers may have circumscribed margins, which may be misdiagnosed as benign breast mass or lead to delayed diagnosis in advanced stages of cancer. Most frequently, circumscribed breast cancers are high-grade invasive ductal carcinoma of triple-negative molecular subtype. However, there are many other rare histological subtypes of cancers in the breast with circumscribed margins or less aggressive features on imaging and mimic benign breast lesions. A radiologist needs to be familiar with the imaging features of various atypical malignant breast tumors to avoid delay in diagnosis. These rare malignant breast tumors are mucinous carcinoma, papillary carcinoma, lymphoma, leukemia, myeloma, metastasis from extramammary primaries, adenoid cystic carcinoma, signet-ring carcinoma, malignant phyllodes tumor, mesenchymal sarcoma, Ewing’s sarcoma, and medullary carcinoma. This pictorial review illustrates the clinical, multimodality imaging features of rare malignant breast tumors with less aggressive features on conventional breast imaging with pathological correlation.
Keywords
Rare breast cancers
rounded breast cancers
breast cancer with circumscribed margins
breast cancer imaging
radio-pathological correlation of rare breast cancer
Introduction
A well-circumscribed breast lump without microcalcifications and architectural distortion is primarily benign. However, a small percentage (10-20%) of patients can be malignant and mostly high-grade infiltrating ductal carcinoma of triple-negative (estrogen, progesterone, and HER-2/neu receptors negative) molecular subtypes.[1] Many breast cancers with rare histological diagnosis may also present with circumscribed smooth margins, which could be mistaken as benign breast lesions, leading to delayed breast cancer diagnosis and adversely affecting the prognostic outcome. A radiologist needs to be familiar with imaging features of various atypical malignant breast tumors to avoid delay in diagnosis. This article describes and discuss twelve cases of circumscribed malignant breast tumors with rare histological diagnoses.
Mucinous or colloid carcinoma
Mucinous carcinoma is a rare type of primary breast cancer that accounts for about 2% of all invasive breast cancers, commonly seen in older women and considered to have a better prognosis with a 10-year survival of 87-90.4%.[2] It usually displays benign clinical and imaging features, leading to delayed diagnosis. On a mammogram, mucinous carcinomas are oval or rounded low-density or isodense masses with circumscribed or microlobulated margins. Calcifications are rare. On ultrasound, they are typically isoechoic or hyperechoic to the subcutaneous fat with posterior acoustic enhancement or no changes. On color doppler, they are usually hypervascular. Magnetic resonance imaging (MRI) shows variable signal intensity on T1-weighted images, the homogeneously or heterogeneously hyperintense signal on T2-weighted images due to rich mucin content, and heterogeneous or rim enhancement on post-contrast T1-weighted images. Histologically, they demonstrate small clusters of malignant cells floating in the mucin pool [Figure 1].
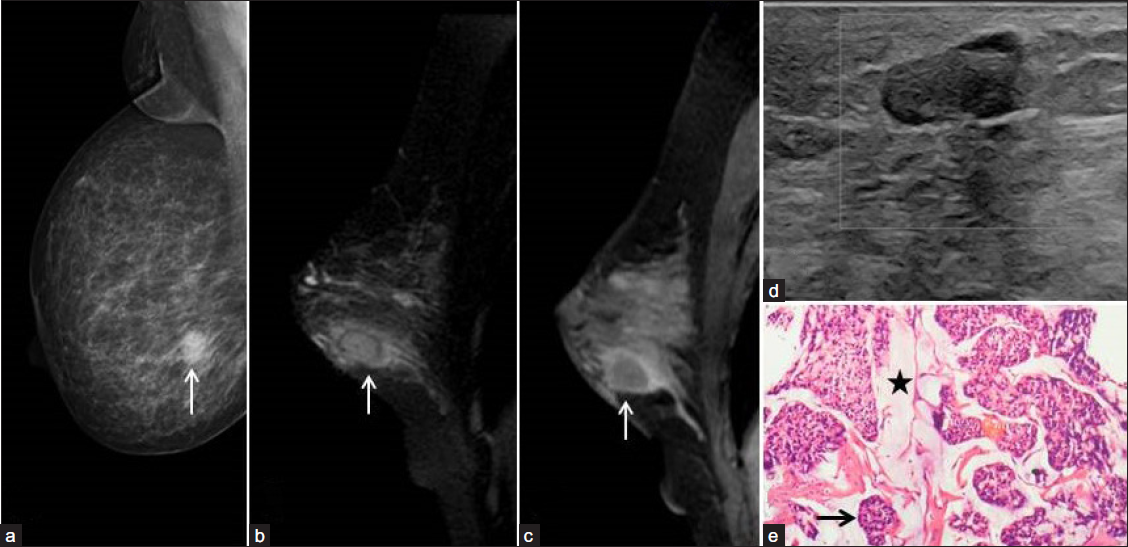
- A 72-year-old woman with a painless right breast lump. Digital mammogram right Medio-lateral oblique view (a) showed a circumscribed high-density ovoid mass in the lower quadrant (arrow). Fat-saturated T2-weighted (b) and post-contrast T1-weighted MRI sagittal (c) sections showed the corresponding mass has a hyperintense signal in the center with hypointense thin enhancing rim (arrow) and perilesional edema (arrow). Ultrasound with color Doppler (d) revealed circumscribed isoechoic mass to subcutaneous fat with no vascular flow. (e) Histopathology of the surgical specimen revealed mucinous carcinoma. H & E-stain 100x showed nests of neoplastic cells (black arrow) floating in the pools of mucin (black star).
Solid papillary carcinoma
Papillary carcinoma accounts for less than 2% of all breast cancers, and solid papillary carcinomas are an even more uncommon entity, more frequently detected in postmenopausal women (>50 years of age). Patients usually present with bloody nipple discharge or a palpable central breast lump.[3] On mammography, solid papillary carcinomas are usually rounded hyperdense masses with circumscribed margins and more commonly located in the retroareolar region. Calcifications within the mass may be seen in 25% of cases. Ultrasound with color doppler is the most sensitive imaging technique, which shows hypoechoic solid mass within a dilated duct, complex intracystic solid mass, or a homogeneous hypoechoic rounded solid mass with a large feeding artery [Figure 2]. On MRI, they appear as enhancing solid mass or complex cysts with enhancing mural nodules.[4] Papillary carcinomas are usually slow-growing tumors and have a favorable prognosis.[5] Solid papillary carcinomas are pathologically characterized by circumscribed rounded nodules composed of low-grade ductal cells separated fibrovascular cores and usually positive for estrogen and progesterone receptors, low proliferation index (ki-67 index), and negative for HER-2/neu. Additionally, they may be positive for neuroendocrine markers such as synaptophysin and chromogranin.[6]

- A 65-year-old woman with a left breast lump. Digital mammogram left craniocaudal and mediolateral oblique views (a, b) showed a rounded hyperdense mass with circumscribed margins in the upper outer quadrant (arrows). Ultrasound with color doppler (c) revealed a hypoechoic circumscribed mass with posterior acoustic enhancement and perilesional vascular flow. Histology (d) revealed solid papillary carcinoma. H & E-stain 100x showed solid tumors with fibro-vascular cores.
Lymphoma
Breast lymphoma is an exceedingly uncommon entity, accounting for only 0.04-0.7% of all breast cancers. It is almost always of Non-Hodgkin’s (NHL) type.[7] Breast lymphoma can be either primary or secondary lymphomatous infiltration of breast tissue. Primary breast lymphoma accounts for 0.85-2.2% of all extra-nodal lymphoma; the patients usually present with rapidly growing palpable breast lumps. Pain may be associated in 25% and axillary lymphadenopathy in 20-40% of patients.[8] Primary breast lymphoma usually presents on mammography as a solitary, circumscribed, rounded, or oval hyperdense mass without calcification or architectural distortion. In the US, a homogeneous hypoechoic mass with distinct smooth margins is found [Figure 3].[9] Mass usually shows rapid initial enhancement and plateau or wash out in the delayed phase (Type II or III kinetic curve) on contrast-enhanced dynamic MRI. On histological examination, diffuse large B cell lymphoma is the most typical type of NHL detected, followed by follicular lymphoma. Similar to other lymphomatous locations, breast lymphoma does not require surgical excision, and chemotherapy is the preferred therapeutic option. The prognosis depends on the stage and histological subtype of breast lymphoma.

- A 60-year-old woman with a painless palpable lump in the right breast. Digital mammogram right CC view (a) showed a circumscribed, oval hyperdense mass in the outer quadrant (arrow). Ultrasound of the corresponding mass (b) showed a homogeneous hypoechoic oval parallel mass (arrow). PET/CT (c, d) showed FDG avid mass (SUVmax 18). Histopathology and immunohistochemistry yielded primary non-Hodgkin’s lymphoma of the breast. H & E stain 400x (e) showed sheets of monotonous population of large atypical cells with nuclear pleomorphism and dispersed chromatin.
Leukemia
Breast involvement due to leukemia is rare, with less than 200 cases reported. Leukemic involvement of the breast can be seen either as an isolated lesion or as a part of systemic disease or as a relapse. Clinically, most cases of breast leukemia usually present as rapidly enlarging, painless, palpable breast lump. Lumps may be bilateral or multiple simulating fibroadenomas. Axillary lymphadenopathy may be seen in 17% of cases.[10] Mammographically, breast leukemias are commonly identified as rounded or oval iso or high-density mass with circumscribed margins. Microcalcifications are exceedingly rare. On ultrasound with color Doppler, they show single or multiple hypoechoic or heteroechoic masses with circumscribed or microlobulated margins, posterior acoustic enhancement and intralesional vascular flow [Figure 4]. On contrast-enhanced MRI, breast leukemia lesions show intense rapid enhancement with washout (Type III kinetic curve). Ninety percent of breast leukemias are T-cell neoplasms; the remaining 10% are B-cell neoplasms. Aspiration biopsy shows blast cells with hyperchromatic nuclei and scanty cytoplasm. The immunohistochemistry (IHC) profile shows positivity for CD19, CD 79a, and CD 22 and variable expression for CD20, CD 34, CD45, and CD 99.[11]

- A 15-year-old girl treated for acute lymphoblastic leukemia (ALL) relapse in the breast. (a, b) A digital mammogram of left craniocaudal and mediolateral oblique projections revealed a rounded isodense mass (thin white arrows) with obscured margins and multiple enlarged left axillary lymph nodes (thick white arrow). Ultrasound (c) with elastography (d) showed a well-circumscribed rounded hypoechoic mass with a hyperechoic rim and elastic score of 5. Histopathology (e) yielded T-cell lymphoblastic leukemic deposits in the breast. H & E 40x showed diffuse compact sheets of monomorphic blast cells with scanty cytoplasm.
Plasmacytoma
Similar to other hematological malignancies, myeloma of the breast is also rare. It may present as primary breast myeloma (plasmacytoma) or as a part of the extramedullary manifestation of disseminated multiple myeloma. Primary breast plasmacytoma is exceedingly rare (0.01%), and about 20% may develop multiple myeloma later in life.[12] Bone marrow biopsy is required to confirm or rule out disseminated multiple myeloma. On mammography, they appear as single or multiple hyperdense masses with circumscribed margins in 50% of cases and without microcalcifications. They are most frequently present on ultrasound as rounded hypoechoic circumscribed masses with posterior acoustic enhancement, intralesional vascular flow, and axillary lymphadenopathy [Figure 5]. The histological hallmark of plasmacytoma is the presence of several atypical plasma cells with irregular nuclei, prominent nucleoli, and scanty marrow plasma cells (<5% of the marrow cells). IHC shows diffuse positivity for the CD138 marker, and 50% of plasmacytomas may express CD31.[13]

- A 40-year-old woman with myeloma left breast. Digital mammogram left cranio-caudal projection (a) showed a well-circumscribed rounded hyperdense mass in the outer quadrant without architectural distortion or calcifications. Ultrasound (b) revealed a mixed echogenic rounded mass with hyperechoic rim, intralesional vascular flow, and posterior acoustic enhancement. Histopathologic examination (c) yielded plasma cell neoplasm myeloma in the breast. H & E-stain 40x showed sheets of plasma cells with atypical morphology and few binucleate forms. (d, e) Immunohistochemical stain showed strongly positive CD138 and negative LCA markers.
Metastasis
Breast metastases from extramammary primary are rare, with an incidence rate of 1.3% to 2.7%.[14] The common primary malignancies that can metastasise to the breast are melanoma, lung, ovarian, and renal carcinomas. Clinically, they present with unilateral palpable painless breast lump, commonly located in the upper outer quadrant. On mammography, breast metastases from the extramedullary primary are usually single (85%), rounded or oval, isodense or high density masses with circumscribed margins without architectural distortion and calcifications. On ultrasound, hypoechoic or mixed echogenic rounded or oval mass with posterior acoustic enhancement [Figure 6]. Axillary lymphadenopathy is unusual. Histological diagnosis of breast metastases is more difficult in an occult extramammary primary malignancy. However, the clinical details and specific immunohistochemical markers may help in the differentiation of primary breast cancer (hormone receptor, gross cystic fluid protein-15, and CK 7 positive) and metastases from extra-mammary primary (e.g., S100, HMB-45, melan-A for melanoma).[15]

- A 32-year-old woman with metastasis in the breast as the initial presentation of anal melanoma. Digital mammogram, right cranio-caudal view (a) showed a well-circumscribed small hyperdense mass in the inner quadrant (arrow). On ultrasound (b), the corresponding mass (arrow) was ovoid and hypoechoic (size 1.7 cm) with no posterior acoustic changes, suggesting BI-RADS 3 mass, probably fibroadenoma. Three months later, the Mammogram right CC view (c) and US (d) showed the mass had increased in size with an additional nodule (arrows), upgraded to BIRADS 4. CECT chest (e) and pelvis (f) showed an enhancing rounded mass in the right breast (arrow in e) and an eccentric enhancing mass in the anorectum (star in f). Histopathology (g) yielded melanoma metastasis in the breast from anorectal primary. H & E-stain 4x showed a tumor with dense melanin pigment in the breast tissue.
Adenoid cystic carcinoma
Adenoid cystic carcinoma (ACC) of the breast is rare, with an incidence of less than 0.1-0.4% of all breast cancers.[16] Unlike ACC of the salivary gland, ACC of the breast is usually a low-grade tumor and has a very good prognosis with low recurrence rate. Axillary node metastases are rare. Mammographically, ACCs have been described as either focal or developing asymmetric densities or iso to hyperdense mass with circumscribed margins without associated calcifications.[17] On ultrasound, they are hypoechoic or heteroechoic, oval or rounded masses with circumscribed margins. On color Doppler, peripheral vascular flow may be appreciated. On MRI, mass shows variable signal intensities on T1 and T2-weighted images and rapid heterogeneous enhancement with washout on dynamic post-contrast imaging. Histopathology shows an adenoid cystic pattern with large and small cribriform mucinous tumor nests around the tumor stroma [Figure 7].

- A 66-year-old woman with adenoid cystic carcinoma (ACC) presented with a rapidly progressive painless right breast lump with stretched skin. Digital mammogram right Medio-lateral view (a) showed a large, rounded, well-circumscribed hyperdense mass. US of the mass (b) revealed an 8 cm heterogeneous hypoechoic mass with multifocal intralesional small cystic areas. The surgical specimen's histologic examination (c) yielded adenoid cystic carcinoma. H & E-stain 40x showed an infiltrating tumor composed of basaloid cells in cribriform architecture and basophilic basement membrane-like material in spaces. On IHC examination (images not provided), the mass showed ER/PR positive, GATA3 positive, E-Cadherin positive, and CDX2 negative, confirming the primary breast ACC diagnosis.
Signet ring cell carcinoma
Signet ring cell carcinoma (SRCC) is a mucin-producing adenocarcinoma, which can be seen originating from the stomach, colon, lung, breast, and prostate. SRCC of the breast is rare, with incidence ranging between 2% and 4.5%.[18] It can be either primary breast SRCC or metastasis from extramammary SRCC, which can be differentiated based on immunohistochemical markers. Primary breast SRCC is rare, reported as isolated cases or small case series. Like extramammary SRCCs, breast SRCCs are aggressive tumors that frequently metastasize to regional lymph nodes, peritoneum, ovaries, and lungs: Mammographically, isodense or hyperdense rounded or oval mass with circumscribed margins without architectural distortion and microcalcifications. On ultrasound, hypoechoic or mixed echogenic rounded or oval mass with posterior acoustic enhancement increased vascular flow and was frequently associated with axillary lymphadenopathy. Histopathology shows features of signet-ring cells with mitosis and nuclear atypia [Figure 8]. Primary breast SRCC shows positive MUC1, CK7, estrogen receptor, E-cadherin, GATA3, CK20, and negative CDX2.[19]

- A 64-year-old woman with signet ring cell carcinoma. Digital mammogram left medio-lateral oblique view (a) showed an oval circumscribed hyperdense mass in the posterior third of the breast (arrow). Ultrasound of the corresponding mass (b) revealed a hypoechoic parallel mass (arrow) with intralesional vascular flow and no posterior acoustic changes. Histologic examination (c) yielded carcinoma with signet ring differentiation. H & E 40x showed tumor cells in diffuse sheets with vacuolated cytoplasm and eccentric nuclei, rendering a signet ring morphology.
Malignant phyllodes
Malignant phyllodes are rare fibroepithelial neoplasms of the breast, representing about 0.3-0.9% of all breast cancers.[20] Clinically, it presents as a rapidly growing, painless, firm breast mass in women between 35 and 55. Axillary lymphadenopathy is unusual. On imaging, phyllodes tumors closely mimic fibroadenomas and may delay the diagnosis of malignancy. Mammographically, circumscribed, hyperdense mass with lucent halo, and may have associated microcalcifications. On ultrasonography, they are mixed echoic or hypoechoic, rounded mass with circumscribed or microlobulated margins, intralesional cysts, and clefts with posterior acoustic enhancement. On color doppler, mass frequently displays increased vascular flow [Figure 9]. Histologically, they demonstrate hypercellular stromal tissue and nuclear atypia with a high mitotic index. The treatment is surgical excision of the mass with margins exceeding 1 cm as the risk of recurrence is high.

- A 58-year-old woman with a malignant phyllodes tumor. Digital mammogram, left MLO view (a) showed a large rounded hyperdense mass in the posterior third of the breast overlying pectoralis muscle. ultrasound with color Doppler (b) of the corresponding mass showed a mixed echoic rounded mass with posterior acoustic enhancement and hypervascularity within the mass. PET/CT showed homogeneously enhancing mass abutting pectoralis muscle (c) with intense FDG-avidity with SUVmax18.4 (d). Histopathology (e) of the surgical specimen revealed a malignant phyllodes tumor. H & E-stain 40x showed biphasic (epithelial-stromal) components with hypercellularity and atypia.
Liposarcoma
Mesenchymal primary breast sarcomas are rare, accounting for less than 1% of all breast cancers, and liposarcoma accounts for 0.3% of breast sarcomas.[21] Clinically, liposarcoma presents as a palpable breast mass which may closely mimic benign lesion on mammography as circumscribed, rounded or oval mass in the anterior third of breast. Calcifications may be present within the mass. Ultrasonographically, hyperechoic or mixed echogenic, oval, parallel mass with intralesional vascularity. On CT scan, isodense mass to muscle with hypodense fat density and variable enhancement after contrast enhancement [Figure 10]. On MRI, heterogeneous signal with high-intensity areas on T1 and T2WI and suppression of signal on fat saturation sequence and heterogeneous enhancement after contrast enhancement. Histology shows lobules of adipocytes with atypical stromal cells. Liposarcomas in the breast are usually well-differentiated/low-grade cancer and exhibit favorable outcomes compared to other sarcomas. The treatment is surgical excision.

- A 63-year-old woman with breast liposarcoma. Digital mammogram left craniocaudal view (a) showed an oval hyperdense mass with circumscribed margins in the inner quadrant anterior third of the breast (arrow). Ultrasound (b) showed heterogeneous hyperechoic circumscribed parallel mass (arrows). Axial section contrast-enhanced CT scan (c) showed the circumscribed rounded isodense mass to muscle with central fat density (-38 HU) in the inner quadrant of the left breast (arrow). Histologic examination (d) yielded well-differentiated liposarcoma. H & E-stain 400x showed lobules of adipocytes with interspersed atypical stromal cells exhibiting a high nuclear-cytoplasmic ratio with hyperchromasia.
Ewing’s sarcoma
Extraskeletal Ewing’s sarcoma (EES) is rare (reported as isolated case reports). It is a malignant soft tissue tumor occurring in adolescents and young adults between 10 and 30 years of age. Clinically, the patient presents with a rapidly progressing painless palpable breast lump with a high recurrence rate. Imaging modalities help in diagnosis, but the findings are non-specific. They are usually seen as a large circumscribed hyperdense mass without calcifications on mammography and on ultrasound, circumscribed homogeneous or heterogeneous hypoechoic mass with posterior acoustic enhancement. CT and MRI help identify the fat plane with the chest wall, usually preserved in ES arising from the breast [Figure 11]. Contrast-enhanced CT shows heterogeneous enhancement with non-enhancing hypodense areas of necrosis. PET/CT shows intense FDG activity in the enhancing regions. MRI shows low to isointense signal to muscle on T1-weighted images, hyperintense on T2-weighted images, and heterogeneous enhancement after gadolinium contrast administration. Histopathology shows malignant round cells, and immunohistochemistry confirms the diagnosis by demonstrating CD-99 membrane and FL1 nuclear positivity.[22]

- A 45-year-old woman with Ewing’s sarcoma of breast presented with a rapidly progressing painless palpable right breast lump. Digital mammogram right cranio-caudal view (a) showed a well-circumscribed rounded hyperdense mass in the medial breast. PET/CT (b and c) showed a large rounded mass with multifocal enhancing solid areas with intense FDG activity (SUV max 16.6). Histologic and immunohistochemical examination of the surgical specimen revealed Ewing’s sarcoma. H & E-stain 400x (d) showed sheets and clusters of small round cells with scant cytoplasm and hyperchromasia.
Medullary carcinoma
Medullary carcinoma of the breast is another rare invasive breast malignancy and accounts for less than 5% of all breast cancers.[23] Compared to patients with infiltrating ductal carcinoma, medullary carcinoma patients tend to be relatively young and have a better prognosis with 10-year survival rates up to 84.[24] Grossly, medullary carcinoma is usually a large distinct mass, firm in consistency with a circumscribed margin. The larger masses may have cystic change within. Medullary carcinoma can be mistaken clinically and radiologically for benign breast masses, e.g., fibroadenoma.[25]
Medullary carcinoma is usually a non-calcified mass with indistinct or circumscribed margins on mammography and a circumscribed mass with an inhomogeneous hypoechoic echotexture on ultrasound examination [Figure 12].[26] On MRI, medullary carcinomas are usually oval or round-shaped masses with circumscribed margins and show rim enhancement of plateau or washout pattern on a kinetic curve with or without enhancing internal septations.[27]

- A 54-year-old woman with medullary carcinoma of the breast presented with mild discomfort in her right breast. Digital mammogram right craniocaudal view (a) showed a circumscribed high-density mass (white arrow) in the outer quadrant without microcalcifications or architectural distortion. Breast ultrasound (b) showed a circumscribed, hypoechoic, rounded mass (white arrow) without posterior acoustic pattern. Contrast-enhanced breast MRI (c) showed a rim enhancing rounded mass (white arrow) with central non-enhancing necrosis or cystic changes. Histological examination (d) showed triple negative invasive breast carcinoma with medullary features.
Conclusion
Breast malignancies with circumscribed margins and rounded or oval shapes are not always benign. Many unusual breast cancers can present with less aggressive features on imaging and mimic benign breast lesions such as fibroadenomas. Hence, it is essential to be aware of rare breast cancers' clinical and imaging features to avoid misdiagnosis or delayed diagnosis. All breast masses should be carefully assessed on imaging for other associated and subtle features such as mammogram calcifications, microlobulated margins and axillary lymphadenopathy on the US, intralesional vascular flow on color doppler, and lesion stiffness on elastography. Contrast-enhanced breast MRI may be added to characterize the functional as well as morphological aspects of the mass in more detail, which would be further helpful in the differentiation of benign and malignant breast masses. Core biopsy and immunohistochemical examinations are essential for diagnostic confirmation and therapeutic planning; hence, they should always be recommended in circumscribed breast masses with any degree of suspicion on imaging.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Can MR Imaging contribute in characterizing well-circumscribed breast carcinomas Radiographics. . 2010;30:1689-1702.
- [Google Scholar]
- MRI features of mucinous cancer of the breast: correlation with pathologic findings and other imaging methods. Am J Roentgenol. 2016;206:238-246A.
- [Google Scholar]
- Breastintracystic papillary carcinoma: an update. Breast J. 2009;15:639-644.
- [CrossRef] [PubMed] [Google Scholar]
- Intracystic papillary carcinoma of the breast. AJR Am J Roentgenol. 2003;181:186.
- [CrossRef] [PubMed] [Google Scholar]
- Intracystic Papillary Carcinoma of the Breast: An In Situ or Invasive Tumor? Results of Immunohistochemical Analysis and Clinical Follow-up. Am J SurgPathol. 2011;35:1-14.
- [Google Scholar]
- Non-Hodgkin’s lymphoma of the breast: a review of 18 primary and secondary cases. Ann DiagnPathol. 2006;10:144-8.
- [Google Scholar]
- Lymphomas involving the breast: a study of 106 cases comparing localized and disseminated neoplasms. Am J SurgPathol. 2008;32:1299-1309.
- [Google Scholar]
- Breast lymphoma: imaging findings of 32 tumours in 27 patients. Radiology. 2007;245:692-702.
- [CrossRef] [PubMed] [Google Scholar]
- Extramedullary B lymphoblastic leukemia/lymphoma (B-ALL/LBL): A diagnostic challenge. Clin Lymphoma Myeloma Leuk. 2014;14:e115-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Breast myeloma: a report of 3 cases with fine needle aspiration cytologic findings. Acta Cytol. 2005;49:445-448.
- [CrossRef] [PubMed] [Google Scholar]
- CD31 (JC70) expression in plasma cells: an immunohistochemical analysis of reactive and neoplastic plasma cells. J Clin Pathol. 1997;50:490-493.
- [CrossRef] [PubMed] [Google Scholar]
- Breast metastasis as initial presentation of asymptomatic gastroesophageal carcinoma: A case report. Indian J Cancer. 2019;56:370-1.
- [CrossRef] [PubMed] [Google Scholar]
- Histological features useful in the distinction of phyllodes tumour and fibroadenoma on needle core biopsy of the breast. Histopathology. 2007;51:336-344.
- [CrossRef] [PubMed] [Google Scholar]
- Adenoid cystic carcinoma of the breast. AJR Am J Roentgenol. 2010;194:1391-6.
- [CrossRef] [PubMed] [Google Scholar]
- Adenoid cystic carcinoma of the breast: mammographic appearance and pathologic correlation. AJR Am J Roentgenol. 1998;171:1679-1683.
- [CrossRef] [PubMed] [Google Scholar]
- Signet-ring cell carcinoma of the breast. Pathol Int. 2000;50:67-70.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical Characterization of Signet-Ring Cell Carcinomas of the Stomach, Breast, and Colon. American Journal of Clinical Pathology. 2004;121:884-892.
- [CrossRef] [PubMed] [Google Scholar]
- Liposarcoma of the breast, review of the literature and a report of a case. Jpn J Surg. 1981;11:381-384.
- [CrossRef] [PubMed] [Google Scholar]
- Primary extraskeletal Ewing’s sarcoma/primitive neuroectodermal tumor of breast. Indian J Radiol Imaging. 2016;26:226-230.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Host resistance and survival in carcinoma of breast: a study of 104 cases of medullary carcinoma in a series of 1,411 cases of breast cancer followed for 20 years. BMJ. 1970;3:181-188.
- [CrossRef] [PubMed] [Google Scholar]
- Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow-up. Cancer. 1977;40:1365-1385.
- [CrossRef] [PubMed] [Google Scholar]
- Medullary carcinoma. In: Rosen PP, ed. Rosen’s pathology (3rd ed). Philadelphia, PA: Lippincott Williams & Wilkins; 2001. p. :449-468.
- [Google Scholar]
- Medullary carcinoma of the breast: mammographic and US appearance. Radiology. 1989;170:79-82.
- [CrossRef] [PubMed] [Google Scholar]
- Medullary carcinoma of the breast: MRI findings. Am J Roentgenol. 2012;198:W482-W487.
- [Google Scholar]








